Pioneering Powerful Life Cures
from Science and Nature
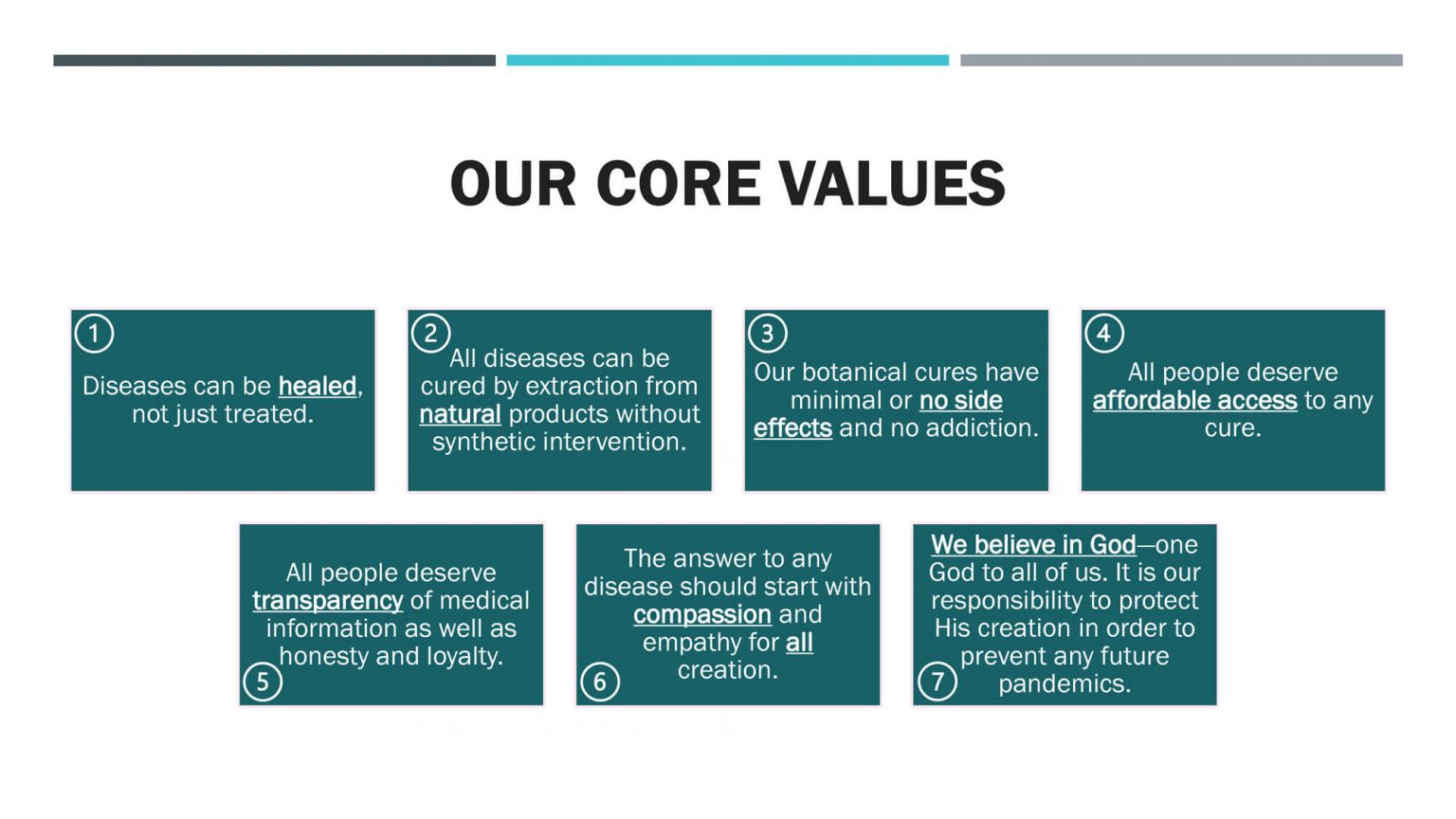
Novel Concepts Medical (NCM)™️ is a medical research and development company founded in 2020 by Rachel R. Alkalay, PhD, to research the role NATURAL compounds can have as POWERFUL solutions for COVID-19 and other serious diseases, which are listed in the Our Medicine sections, such as respiratory diseases, Alzheimer’s disease, cancer, obesity, diabetes, anemia, and more.
Our company is the First in the World to:
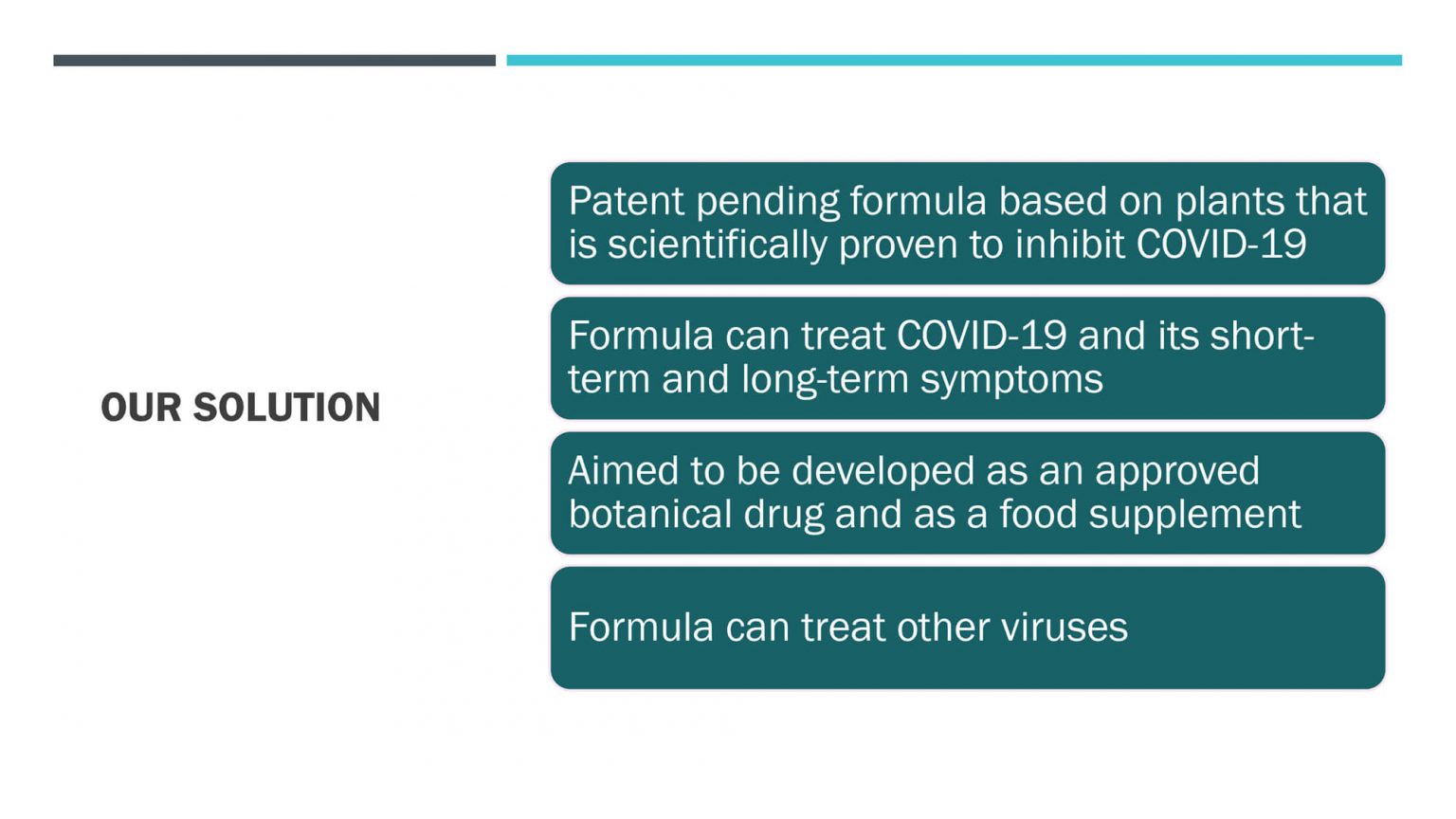
- Succeed in early testing to assist COVID-19 patients to overcome the virus and within 48 hours send us back a negative PCR test result.
- Succeed in blocking the COVID-19 virus’s entry to the cells and stopping its progress, as demonstrated by in vitro testing.
- Succeed in blocking the binding of the Omicron variant into the cells, which means infection by Omicron is blocked.
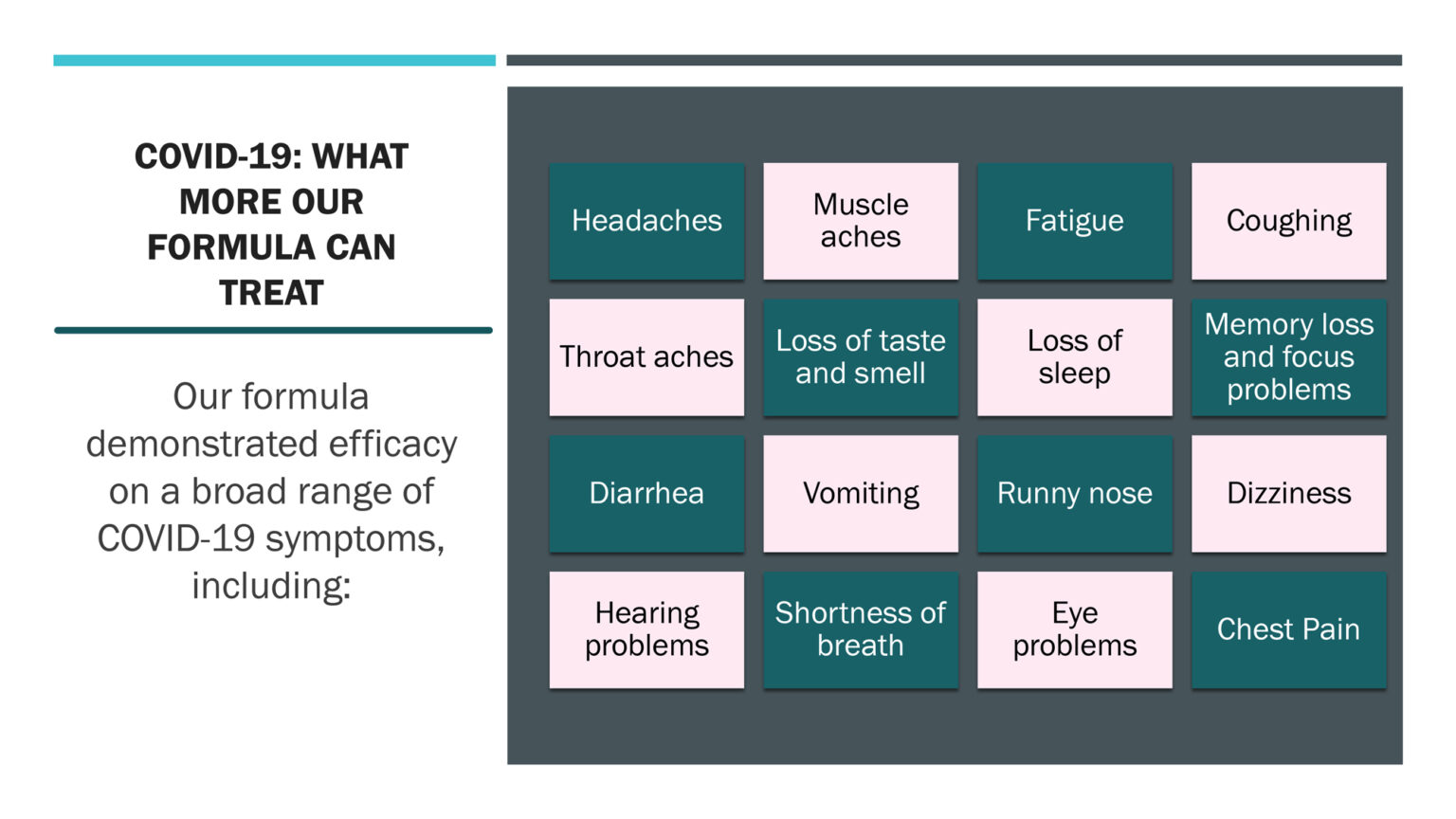
- Succeed in treating more than 16 symptoms of Long COVID-19 with volunteers and either reduce them significantly or eliminate them completely in just a few days.
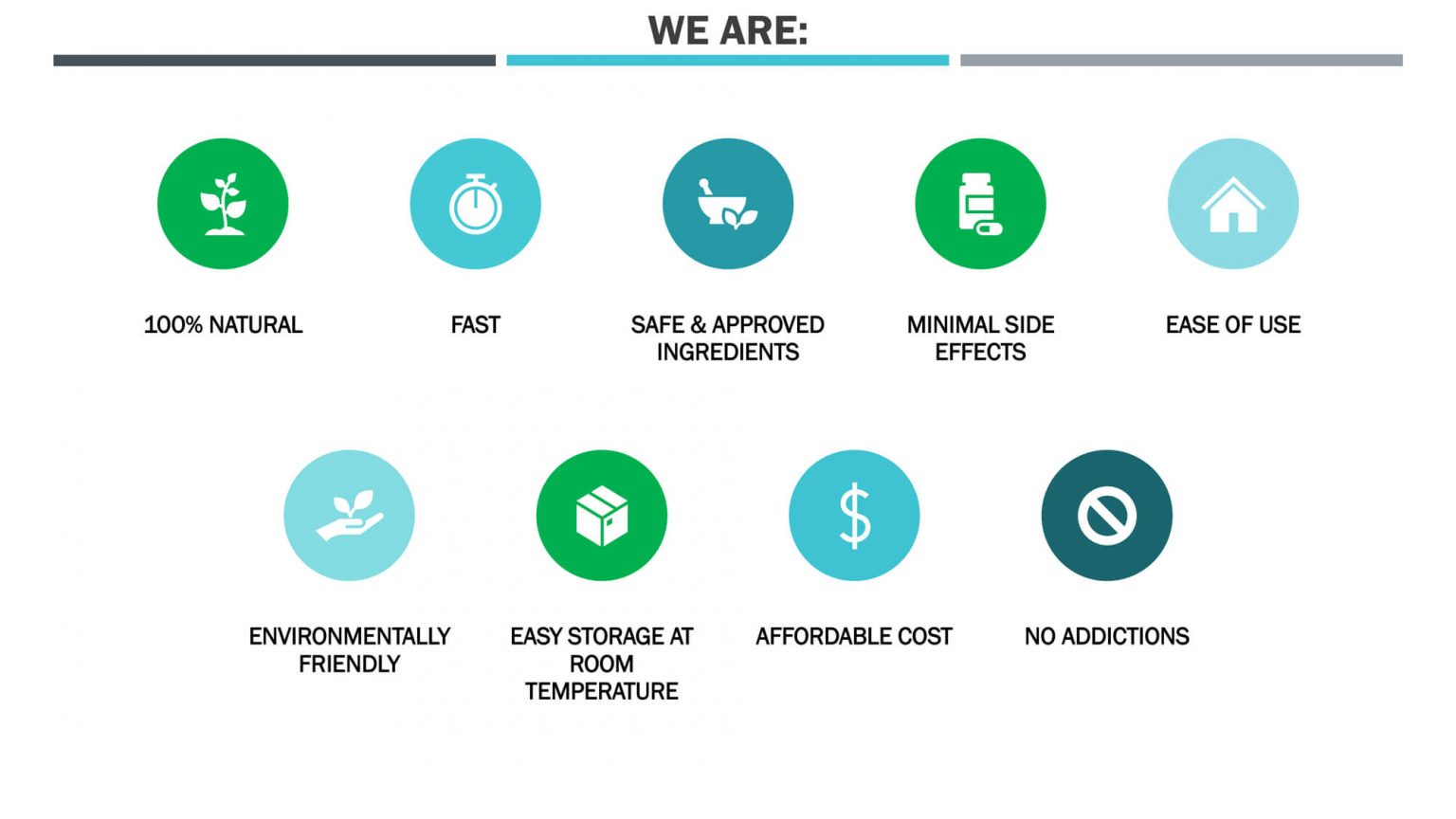
No side effects have been reported by any of our volunteers due to the safety of our plant-based compounds.







Our COVID-19 Cure Lab Reports
Novel Concepts Testing Summary
Production Batch 1
Spike-ACE2 binding disruption
TMPRSS2 Inhibition
Prior to binding with ACE2, the spike protein undergoes priming via a serine protease TMPRSS2. Inhibition of this protease, blocks fusion of the virus with ACE2 and thus represents an additional therapeutic target for COVID-19. The same production batch 1 formulations that were able to disrupt spike RBD – ACE2 binding were also able to inhibit TMPRSS2.
Production Batch 2
Spike-ACE2 binding disruption
None of the production batch 2 formulations demonstrated inhibition of the ACE2 – spike RBD interaction.
TMPRSS2 Inhibition
All of the production batch 2 formulations demonstrated inhibition of the TMPRSS2 protease reaction.
In vitro Antiviral Testing
Three modes were tested for antiviral activity. Each mode added the formulation at a different point in the viral infection process.
In the first mode tested, virus was pre-incubated with the formulation before exposure to the cells. The formulation is not removed when cell infection occurs. All the production batch 2 formulations demonstrated antiviral activity in this test.
In the second mode tested, the cells are pre-incubated with the formulation before viral infection. Two of the production batch 2 formulations tested were effective at inhibiting viral infection in this assay, while the third formulation was not.
In the third mode tested, the cells are infected with virus and then exposed to the formulation. All the production batch 2 formulations demonstrated antiviral activity in this test.
The formulations were active in the three modes which tested exposure at different points during viral infection and replication. Antiviral activity was demonstrated with exposure both before and after viral entry into cells. Both the cellular data and the in vitro enzyme data support the hypothesis that the formulations may target the host cells to prevent viral entry. However, the mechanism of action cannot be definitively determined from these results, and it could be via multiple mechanisms or via one central mechanism.
Overall, these formulations are potential candidates for the treatment of SARS-CoV-2 infections.


